Technology
Overview
What it is
Focused Ultrasound (FUS) is a new neurosurgical technology that involves treating the deep brain in a single outpatient procedure without the need for a surgical incision. Specifically, the technology uses ultrasound waves to create a small ablation to disrupt pathological brain activity in order to reduce the signs or symptoms of Essential Tremor and Tremor-dominant Parkinson’s Disease.
Who can benefit
Currently, FUS is FDA-approved for patients with Essential Tremor and for those with Parkinson’s Disease for whom tremor is the major disabling feature and who have not received satisfactory tremor relief from prescribed medications. Focused Ultrasound (FUS) is FDA-approved for patients with Essential Tremor and Parkinson’s Disease, for whom motor symptoms such as tremor do not respond sufficiently to medications. Initially, treatment is provided for one side of the body. In some cases, an additional treatment for the opposite side can be provided later.
How it works
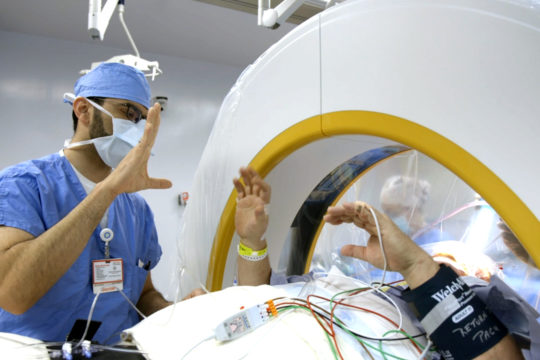
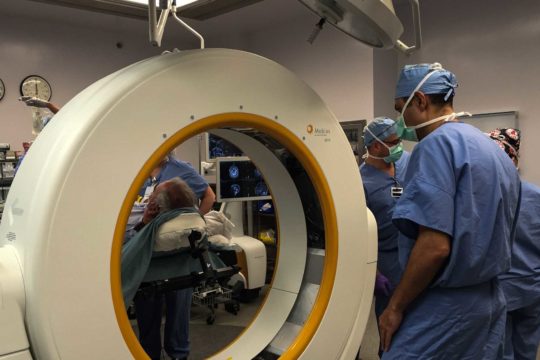
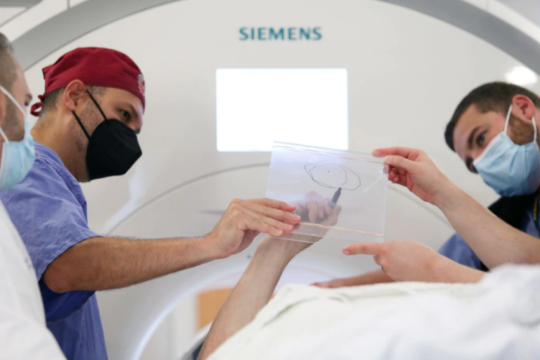
FUS uses accurately focused beams of ultrasound energy to target and heat specific circuits of the brain. The FUS procedure is carried out under near-real-time MRI visualization. A patient lies down on the treatment bed, places his or her head into the FUS helmet and together the patient and helmet are positioned within the MRI scanner. The MRI is able to monitor the temperature of the target location while hundreds of ultrasound transducers in the helmet deliver highly focused energy to that target.
Initially, low energy is delivered to the target to disrupt abnormal brain activity in that circuit. This allows the physician to get feedback from the patient of any potential side effects and adjust the target accordingly. When the tremor is reduced or eliminated, then the temperature is increased so that the target can be ablated, disrupting the circuit responsible for the tremor.
Because ultrasound energy does not travel well through air, patients must shave their heads for the procedure. In addition, because the skull is made up largely of air, if the skull is too thick, the energy cannot be focused accurately, and so some patients may not be eligible for this procedure. A CT scan prior to the procedure will allow us to determine whether it is safe to proceed with MRI-guided FUS.
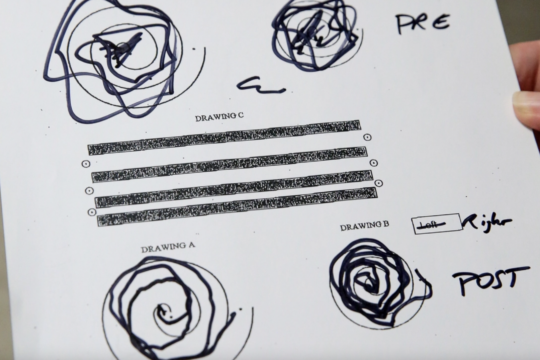
The Archimedes Spiral Test – Before and After FUS Treatment
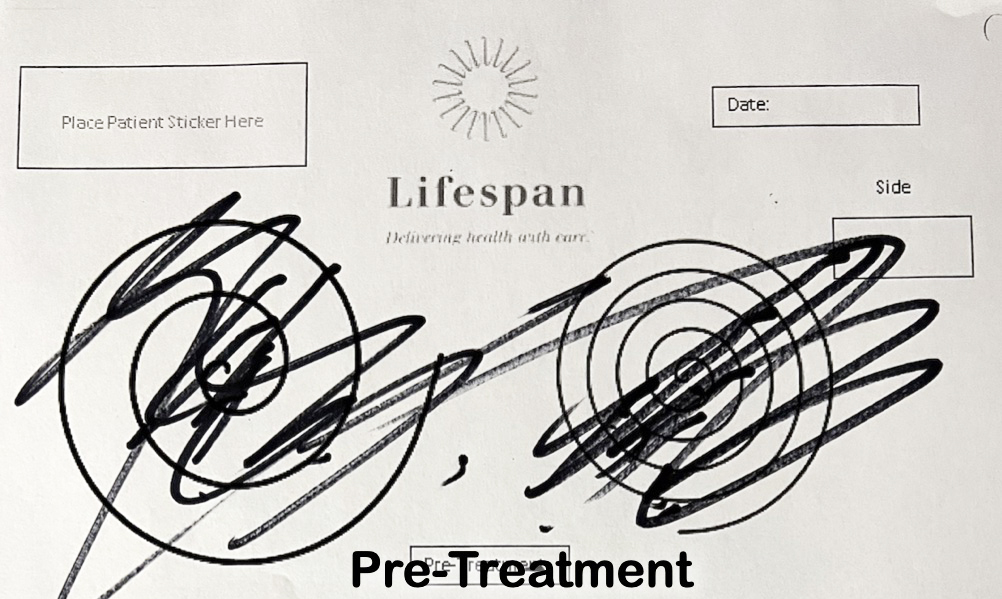
Before FUS Treatment
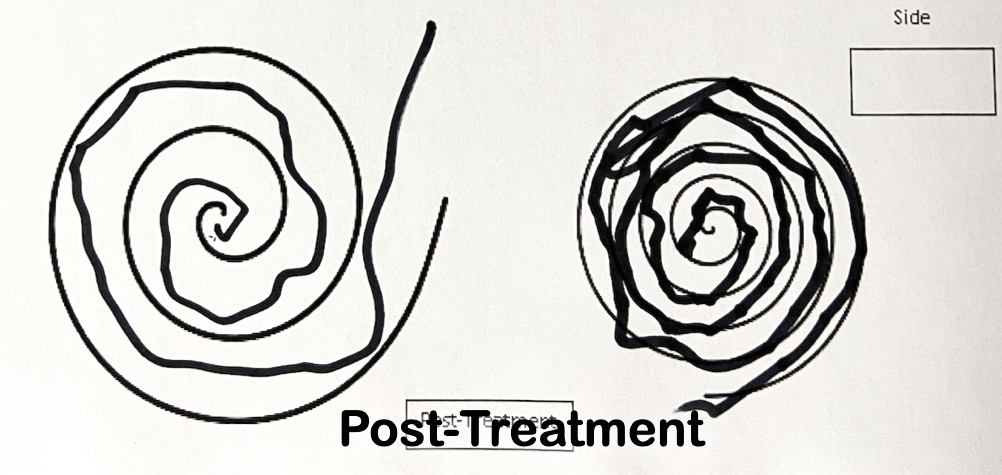
After FUS Treatment
Relevant Conditions
Affiliated Comprehensive Care Center
Contact Information
Rhode Island Hospital
593 Eddy Street
Providence, RI 02909
Phone: 401-793-9134
Fax: 401-444-2788