Regulation of human glioma cell migration, tumor growth, and stemness gene expression using a Lck targeted inhibitor.
J. P. Zepecki, K. M. Snyder, M. M. Moreno, E. Fajardo, A. Fiser, J. Ness, A. Sarkar, S. A. Toms & N. Tapinos
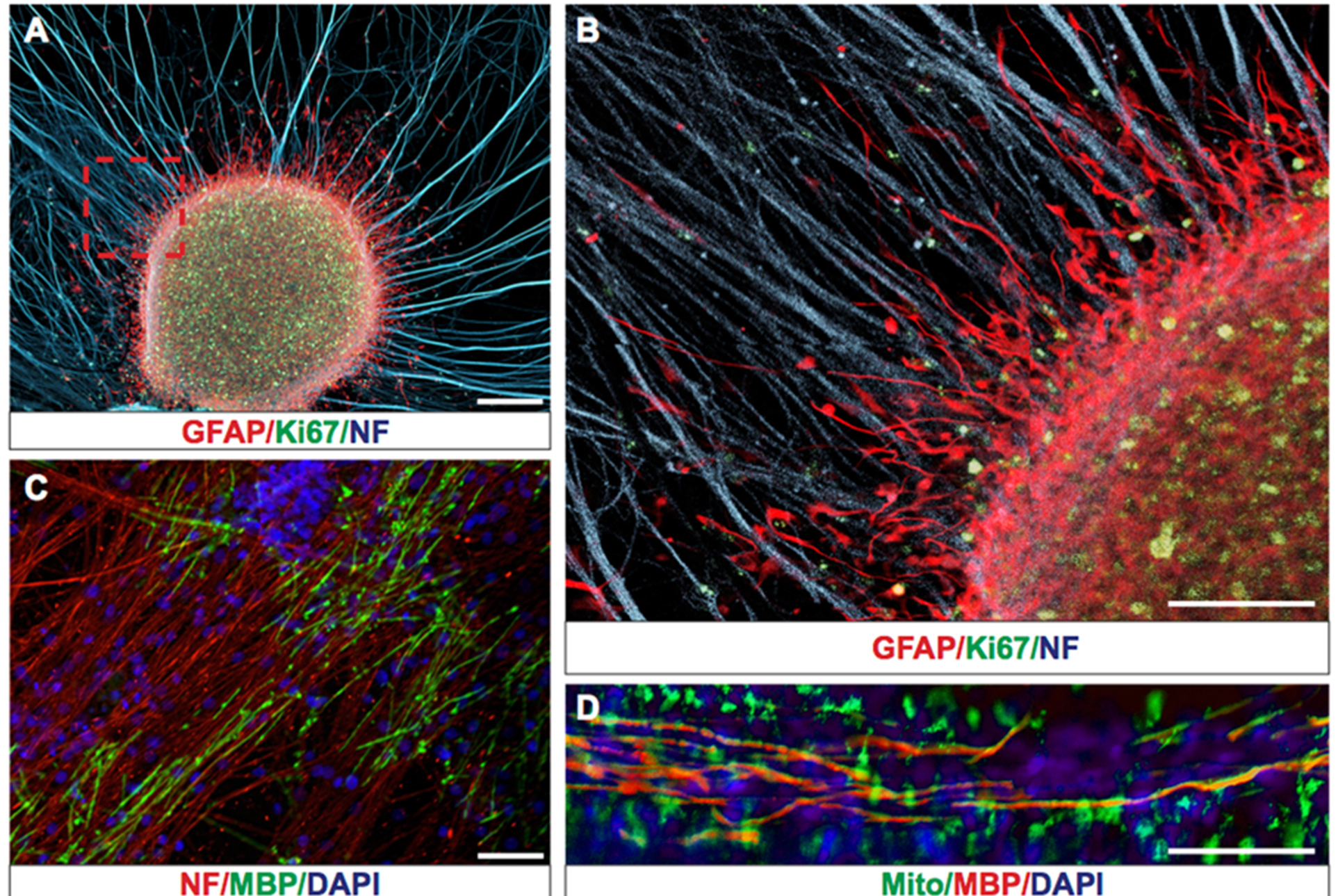
Human glioblastoma is one of the most aggressive and lethal cancers due to the presence of tumor-propagating human glioma stem cells (hGSCs) and the highly migratory nature of these cells [1, 2]. Recent studies have revealed extensive intratumoral heterogeneity in transcript expression, which likely contributes to treatment failure and tumor recurrence [3, 4]. Despite advances in understanding the basic biology of glioblastoma, studies on glioma cell migration are hindered by the lack of efficient in vitro or in vivo migration models. Until now studies on human glioma cell (hGC) migration were performed on Laminin coated surfaces [5], collagen and astrocyte layers [6], extracellular matrix layers [7], electro spun nanofibers [8], or postmortem in mouse xenograft models [7]. Although all these studies have provided information on the migratory properties of hGCs, their real-time interaction with myelinated and non-myelinated axons has not been studied. Migration of hGCs occurs in the brain parenchyma through continuous interaction with axon fibers, glial cells, microglia, and endothelial cells, which likely affect their migratory efficiency and cannot be accounted for with the current monolayer migration models.
Read Full Article: Nature.com/onc/