Isolation and characterization of patient-derived CNS metastasis-associated stromal cell lines
Ben Yi Tew, Christophe Legendre, Gerald C. Gooden, Kyle N. Johnson, Rae Anne Martinez, Jeff Kiefer, Mark Bernstein, Jennifer Glen, Loren Butry, Aleksander Hinek, Steven A. Toms & Bodour Salhia
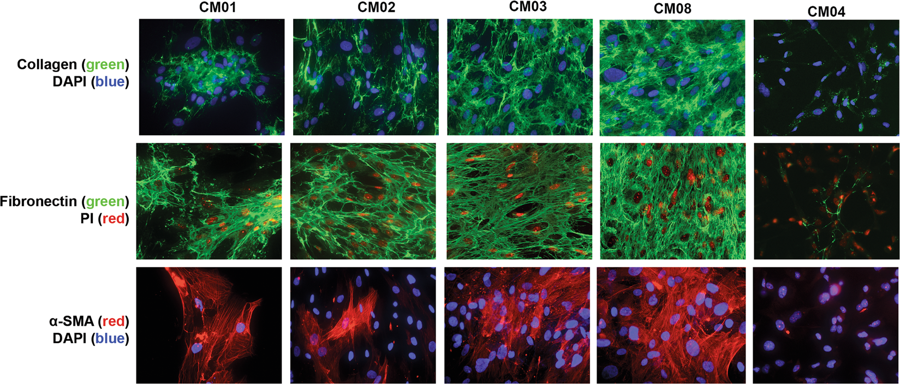
The functional role of human derived stromal cells in the tumor microenviornment of CNS metastases (CM) remain understudied. The purpose of the current study was to isolate and characterize stromal cells of the tumor microenvironment in CM. Four different patient-derived cell lines (PDCs) of stromal and one PDC of tumorigenic origin were generated from breast or lung CM. PDCs were analyzed by DNA/RNA sequencing, DNA methylation profiling, and immunophenotypic assays. The stromal derived PDCs were termed CNS metastasis-associated stromal cells (cMASCs). Functional analysis of cMASCs was tested by co-implanting them with tumorigenic cells in mice. cMASCs displayed normal genotypes compared with tumorigenic cell lines. RNA-seq and DNA methylation analyses demonstrated that cMASCs highly resembled each other, suggesting a common cell of origin. Additionally, cMASCs revealed gene expression signatures associated with cancer associated fibroblasts (CAFs), epithelial to mesenchymal transition, mesenchymal stem cells and expressed high levels of collagen. Functionally, cMASCs restricted tumor growth, and induced desmoplasia in vivo, suggesting that cMASCs may promote a protective host response to impede tumor growth. In summary, we demonstrated the isolation, molecular characterization and functional role of human derived cMASCs, a subpopulation of cells in the microenvironment of CM that have tumor inhibitory functions.
Read Full Article: Nature.com/onc/